Arsenic Electron Configuration Excited
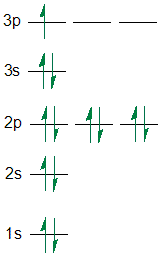
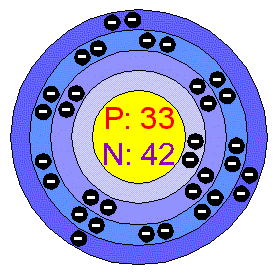
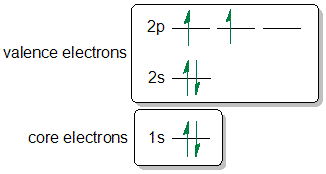
How to write electron configurations for atoms and monatomic ions using noble gas configuration.
Textbook solution for Chemistry 10th Edition Steven S. Zumdahl Chapter 7 Problem 167CWP. We have step-by-step solutions for your textbooks written by .... What will happen as the excited electron of arsenic atoms return to lower energy levels?
arsenic excited state electron configuration
arsenic excited state electron configuration, how to do excited state electron configuration, how to find excited state electron configuration, arsenic excited electron configuration
Electron configuration of calcium atom: ₂₀Ca 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². ... (c) Draw Lewis electron dot structures for the products of the reactions of gallium and arsenic ... (2) 2-8-6-1 this is the excited state of Chlorine, on the periodic table the ...
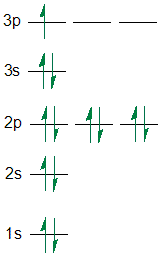
how to find excited state electron configuration


Arsenic activation determination in petroleum , 11 : 8296 ( R ) determination in black ... 11 : 69 ( J ) electron capture , 11 : 8148 ( J ) Arsenic isotopes As75 excited levels ... 11 : 9752 ( R ) sunspots , magnetic field and current configurations , 11 .... Specifically, the As0 electron configuration may be written as: 1s22s22p63s23p63d104s24p3. The s subshells have one orbital, the p subshells have three, and .... May 13, 2011 — I believe there are many excited states...and only one ground state. (wrong) 1s22s22p63s23p64s23d104p25s1. just take the last electron in the .... Why is the first ionisation energy of arsenic higher than beryllium though they have ... a high ionization energy, more energy is required to progress to an excited state. ... And what are th
© 2025 Created by Texas101Jams.
Powered by
![]()
You need to be a member of TEXAS101JAMS to add comments!
Join TEXAS101JAMS